MATHEMATICS
We consider the Hamiltonian of a system of three quantum particles (two identical bosons and a fermion) on the one-dimensional lattice interacting by means of zero-range attractive or repulsive potentials. We investigate the point spectrum of the three-particle discrete Schrödinger operator H(K), K ∈ T which possesses infinitely many eigenvalues depending on repulsive or attractive interactions, under the assumption that the bosons in the system have infinite mass
The present paper is devoted to the problem for one of the loaded wave integro-differential equations, which is equivalent to the nonlocal problem for a higher-order wave equation. The study aims at nonlocal problems and constructs a representation of the solution to the problem for an equation of hyperbolic type. Also, the paper provides examples of some cases where it will be possible to construct solutions to the problem explicitly and in the graphs.
PHYSICS
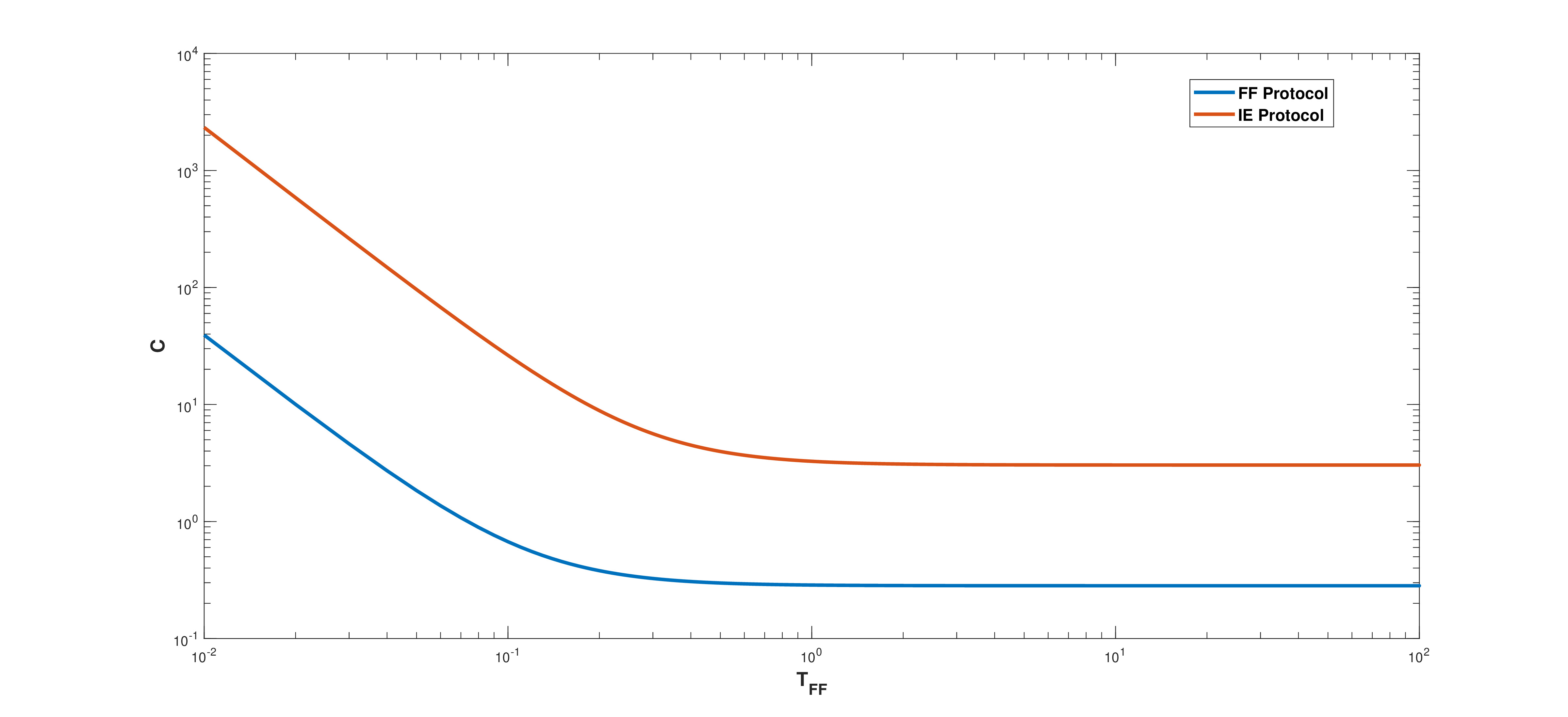
We consider the problem of energy cost needed for acceleration (deceleration) of the evolution of a quantum system using the Masuda–Nakamura’s fast forward protocol. In particular, we focus on dynamics by considering models for a quantum box with a moving wall and harmonic oscillator with time-dependent frequency. For both models we computed the energy needed for acceleration (deceleration) as a function of time. The results obtained are compared with those of other acceleration (deceleration) protocols.
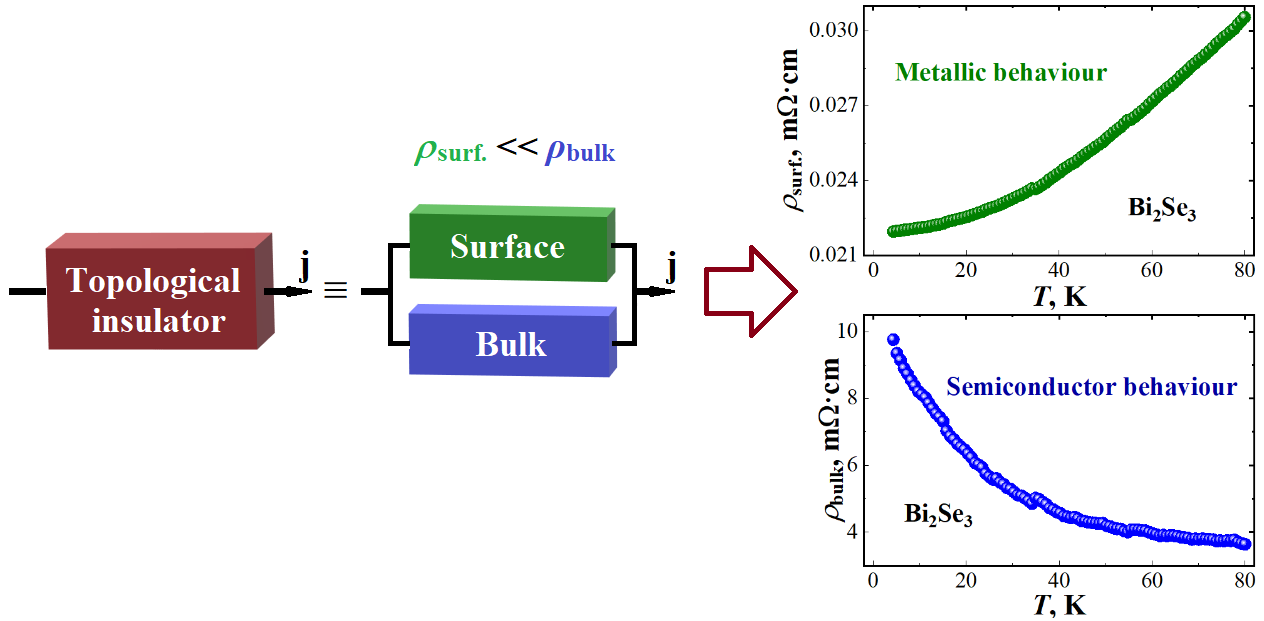
The temperature dependences of the electrical resistivity of topological insulator Bi2Se3 thin films with thicknesses of 20 and 40 nm were measured in the temperature range from 4.2 to 80 K. Their resistivity was shown to depend on thickness. A method was proposed for “separation” of the bulk and surface resistivity of films, with the help of which corresponding estimates were made. It was demonstrated that the surface resistivity is more than two orders of magnitude less than the bulk resistivity at T = 4.2 K.
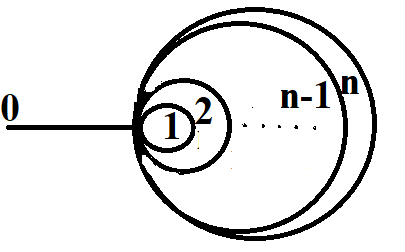
The problem of persistence current in nanosystems is studied. We demonstrate some simple theoretical observation which allows one to construct a benchmark for the persistence current. It can be used for improvement of the persistence current measurement procedure. The consideration is based on the quantum graph model. The benchmark is given by a graph with finite number of rings touching at one point with a lead attached to this point. It is assumed that the graph is plane and there exists a magnetic field orthogonal to the rings.
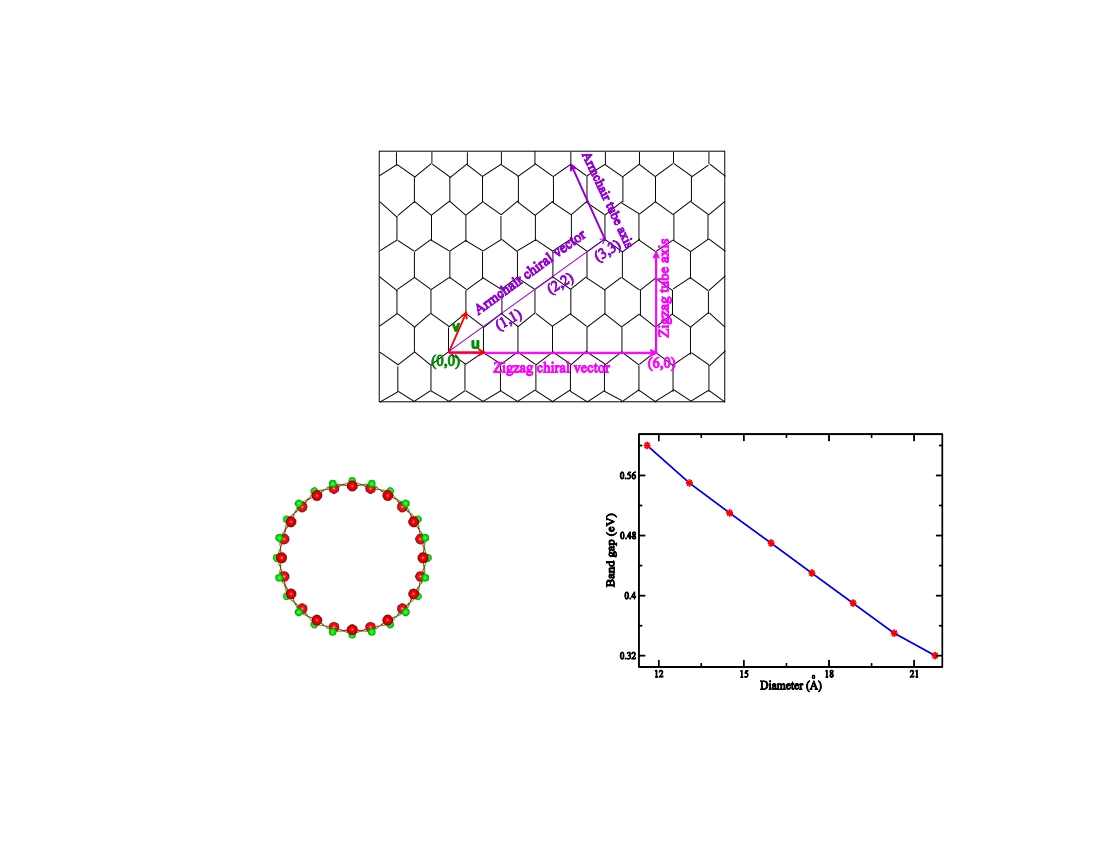
Using density functional theory, evolution of geometrical and electrical properties like wall width, binding energy, strain energy, band structure, density of states etc. of several zigzag HgSe nanotubes with diameters in the range of 11.59 to 21.74 *A are systematically investigated. It is noted that the walls of the nanotubes are gradually becoming thin with increasing tube diameter. This study reveals that the stability of the zigzag HgSe nanotube increases with increasing diameter. It is also perceived that zigzag HgSe nanotubes obey classical elasticity law. Band structure analysis reflects that all the zigzag HgSe nanotubes are direct band gap semiconductors and their band gaps slowly decrease with increasing diameter.
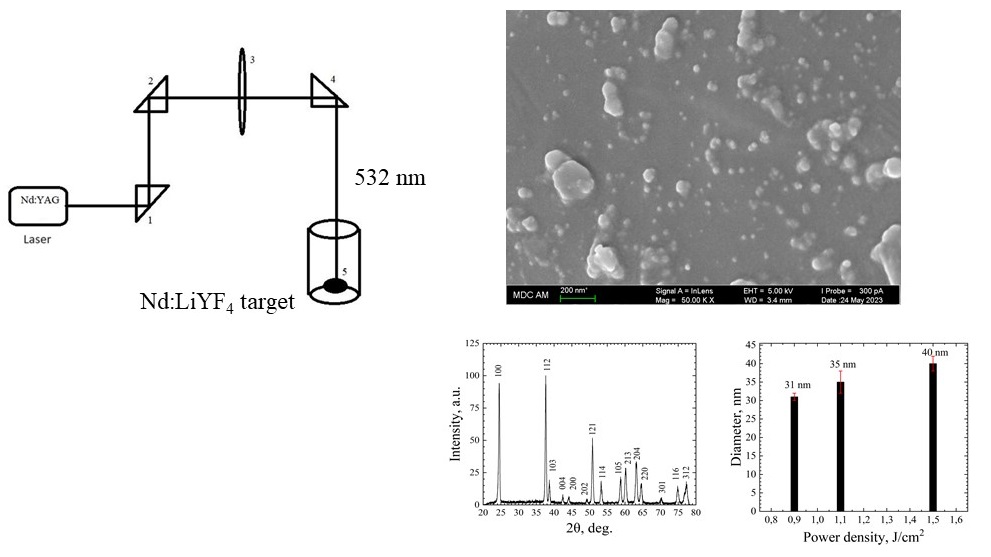
In this work, a set of crystalline LiYF4:Nd3+ nanoparticles with an average size of about 35 nm, were successfully synthesized via laser ablation method in liquid (combination of ethanol and water). The samples have trigonal structure corresponding to LiYF4 host. The spectral and kinetic characteristics of the synthesized nanoparticles corresponded to the characteristics of the target LiYF4:Nd3+ bulk crystal. In particular, the luminescence decay curves for the 4F3/2 →4I11/2 radiative transition (Nd3+) are single-exponential and the decay times were around 517 μs, which is typical for Nd3+ in LiYF4 host. It has been established that the decrease in the power density of laser radiation energy leads to the increase of the average particle size
CHEMISTRY AND MATERIALS SCIENCE
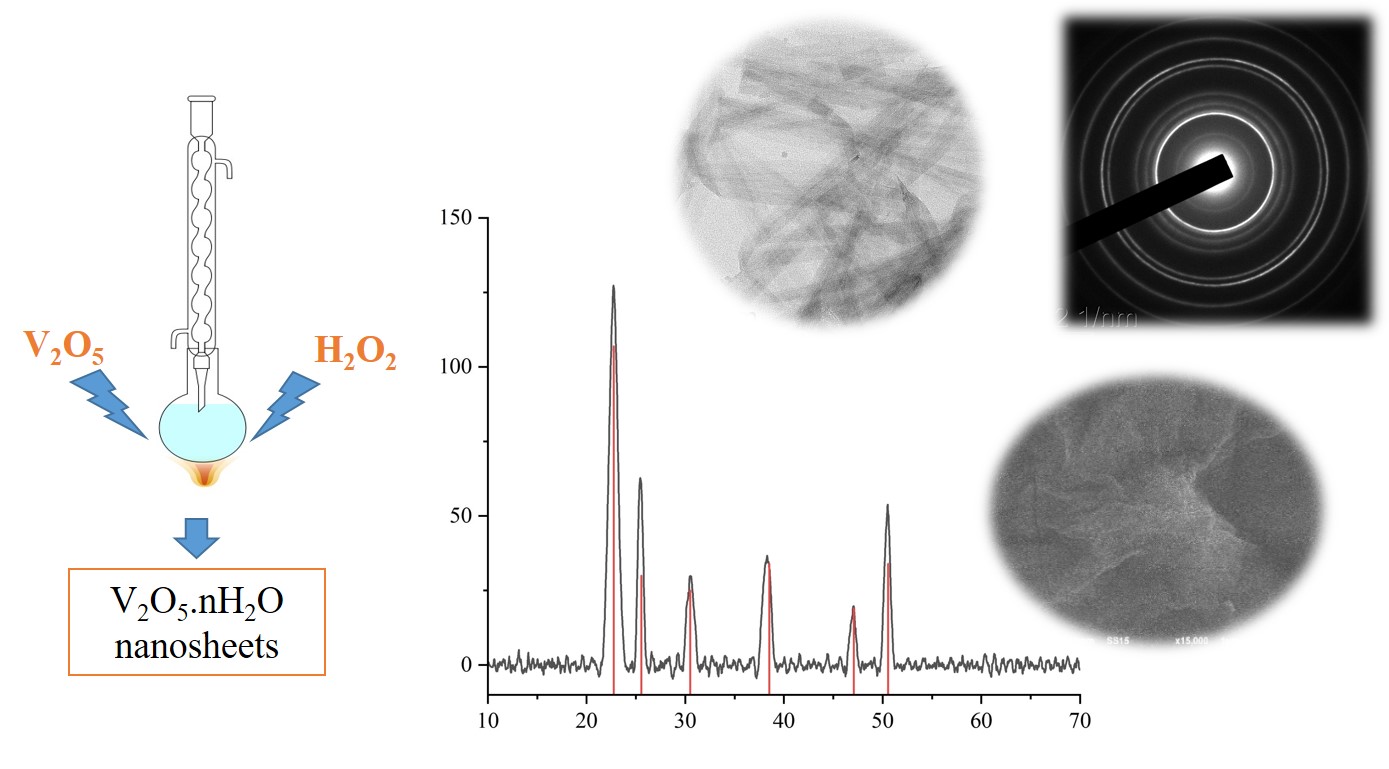
The colloidal properties of the lyophilic dispersion system V2O5 nH2O sol studied in this work was obtained by the thermolysis of V2O5 powder with hydrogen peroxide. The dispersion phase exists in the form of nanorods. The optimal mole ratio of V2O5 and H2O2 for synthesizing the sol is 1:30, and the possible concentration of V2O5 in the entire colloidal system ranges between 0.3 to 1.6 mass percent. The existence of nanoparticles in this colloidal system and the pH range that maintains the stability of the sol conform to the phase diagram of vanadium (V) in an aqueous medium. The absolute value of the zeta potential of the sol increases when the initial concentration of the sol during synthesis increases and the ionic strength of the dispersion medium decreases. Potential curves of pair interaction between nanoparticles were also constructed according to the DLVO theory.
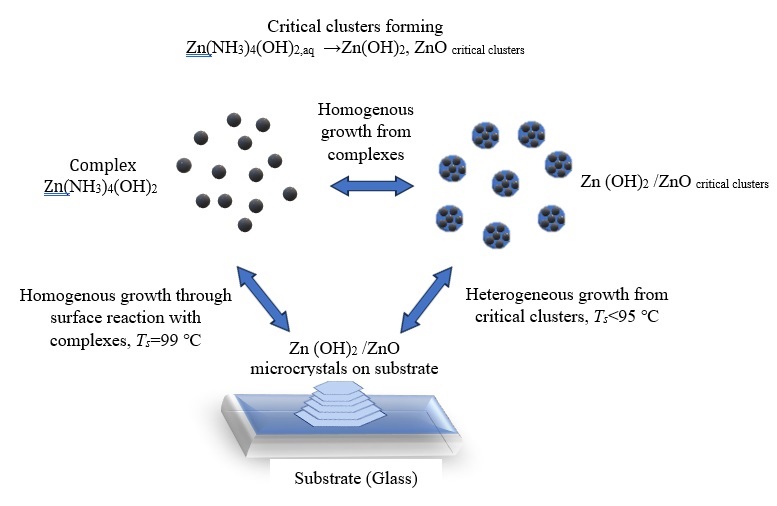
For the closed system (Σ): Zn2+–NH3,aq –NH3,gas, H+–OH−–N2,gas, experimental data on the change in the concentration of in the composition of the ammonia complex Zn(NH3)2+ 4 in solution, colloidal particles Zn(OH)2/ZnO in solution and growing film on the reactor walls are presented depending on the synthesis time, zinc concentration and synthesis temperature Ts in the range of 50 –99 ◦C. It has been established that up to 95 ◦C the ion-molecular growth of Zn(OH)2/ZnO clusters in solution (Σ) proceeds in a diffusion-controlled mode of homogeneous growth until reaching of their critical size. Further growth of ther critical clusters is followed by aggregation and coalescence of critical sized clusters into microcrystals with the formation of a film on a glass substrate of various morphologies. The solubility of such a film is determined by the size of critical clusters, which preserves in the growing polycrystal in the form of coherent scattering region (CSR). With an increase in the synthesis temperature to 99 ◦C, the aggregation mechanism is replaced by a faster diffusion-controlled attachment of Zn (II) ammonia complex to the end surface of the growing microcrystals simultaneously in colloid solution and in the film.
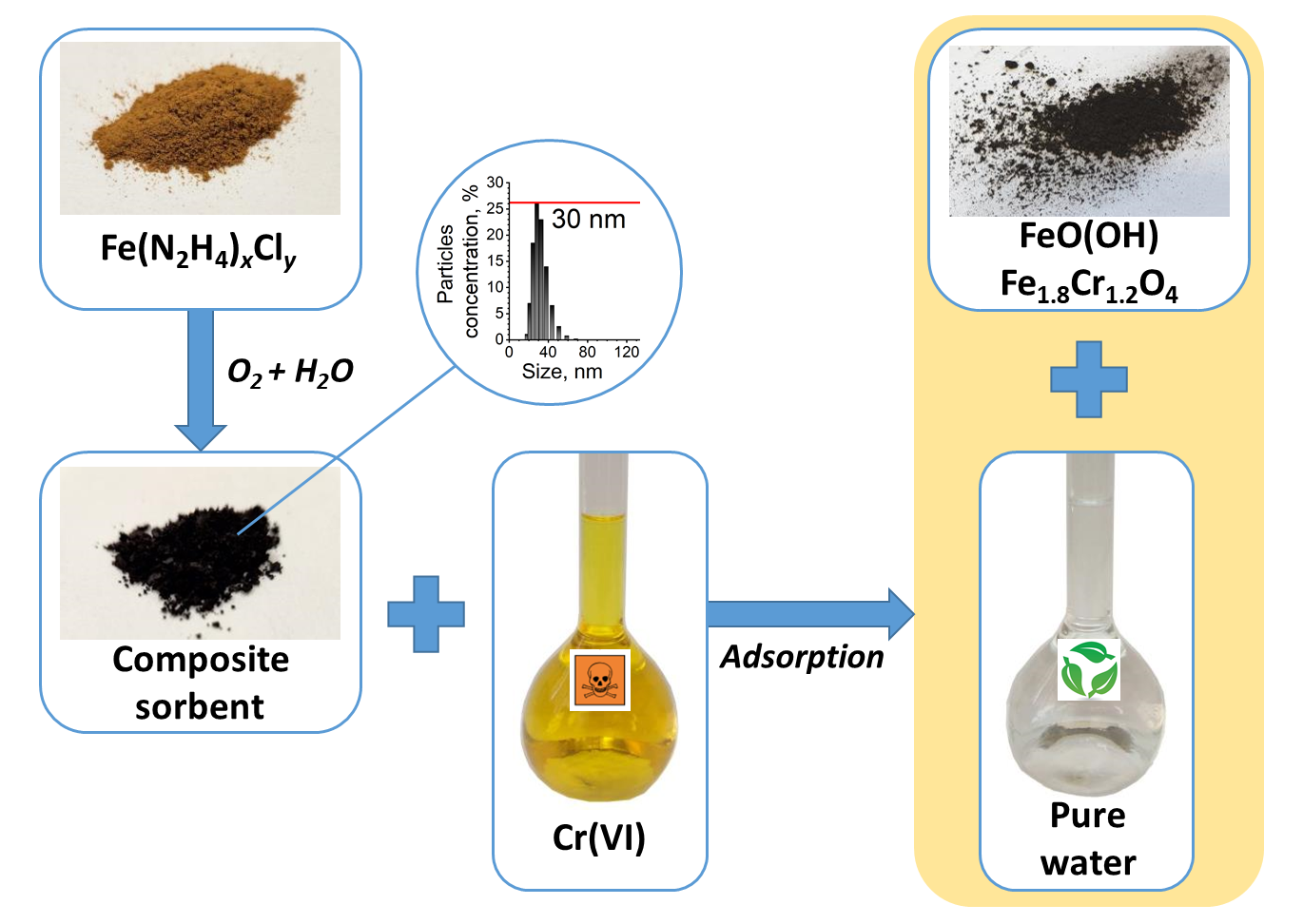
The paper presents a methodology for the synthesis of iron complex with hydrazine hydrate Fe(N2H4)xCly . The Fe(N2H4)xCly complex was investigated by X-ray phase analysis and scanning electron microscopy. Upon hydrolysis, the Fe(N2H4)xCly complex forms a composite sorbent, which is Fe3O4 in a shell of Fe(N2H4)xCly complex. The composite sorbent can be used to treat wastewater from Cr(VI) ions and is effective in the pH range of 2 to 12. Based on the adsorption and electrokinetic potential data, a conclusion about the nature of the terminal groups of the adsorbent was made, a scheme of the structure of its electrical double layer and the adsorption mechanism were proposed. Depending on the conditions, Cr(VI) can be adsorbed on the composite sorbent or reduced to Cr(III). The efficiency of the composite sorbent in the removal of Cr(VI) ions was tested on a sample of real wastewater.
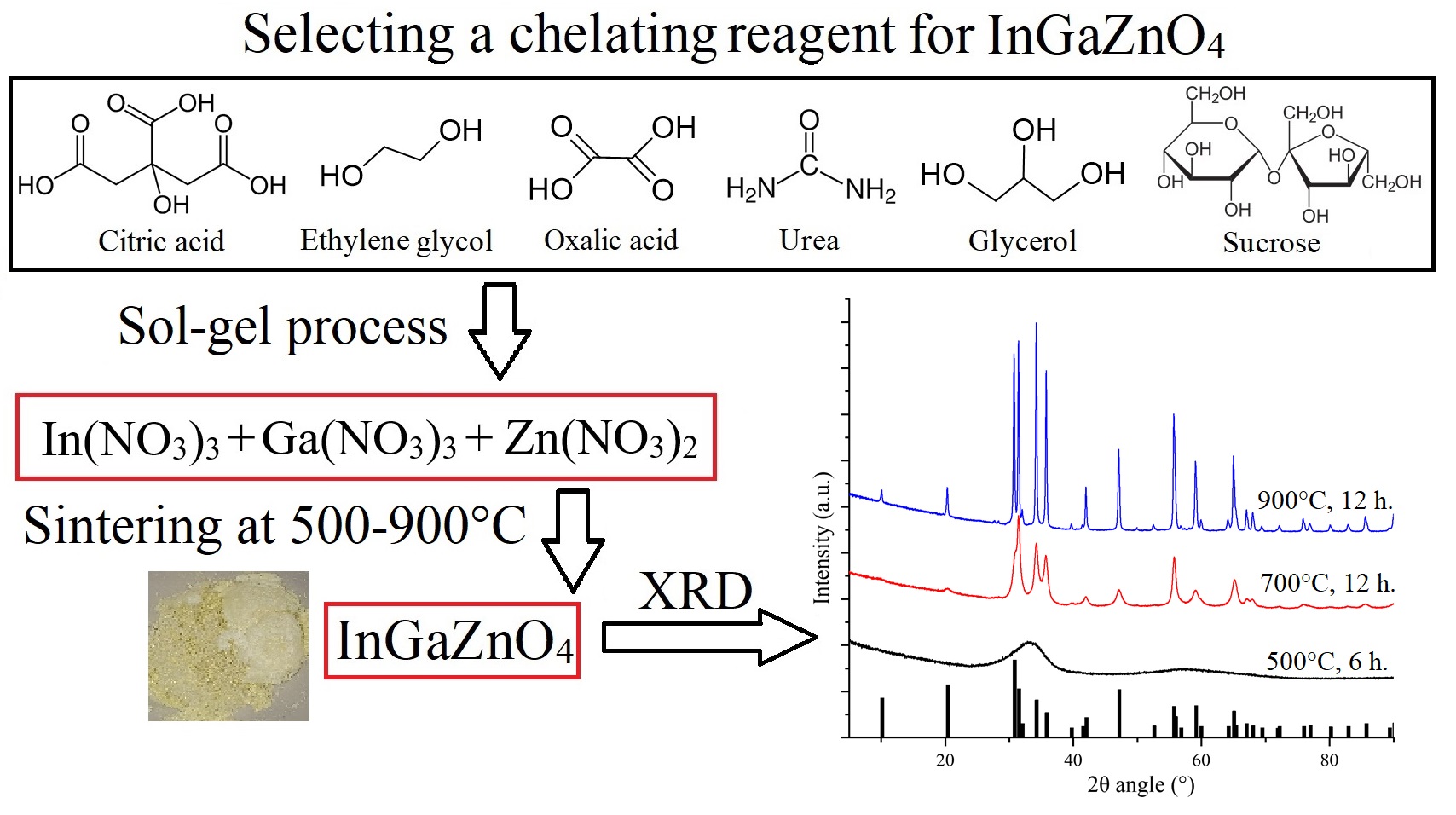
The production of nano-sized semiconductor oxide materials, such as indium-gallium-zinc oxide (IGZO), will make it possible to use it for the transistors manufacture using printing methods. The sol-gel method is one of the widely known and used methods for producing nano-sized oxide materials. As is known, a chelating reagent (complexing agent) can influence both the synthesis process and the final phase composition. The results of sol-gel synthesis with various chelating reagents: citric acid, ethylene glycol, oxalic acid, urea, glycerol and sucrose are presented. The samples were studied by X-ray diffraction. It was found that ethylene glycol and glycerol as chelating reagents make it possible to obtain a homogeneous crystalline material at 900 ◦C with a YbFe2O4-type structure, R-3m (166) space group. Unit cell parameters and crystallite size (Halder-Wagner method) for InGaZnO4 single-phase samples were calculated
Ultradisperse and nanocrystalline powder compositions were obtained in the process of plasma chemical synthesis of a mechanical mixture of titanium carbonitride TiC0.5N0.5 with metallic nickel and molybdenum in a low-temperature nitrogen plasma (4000 – 6000 ◦C), taking into account recondensation in a turbulent flow of nitrogen gas. It was established by X-ray diffraction that their phase composition is characterized by the presence of cubic compounds in the form of titanium-molybdenum carbonitride Ti0.8Mo0.2C0.5N0.5, metallic Ni and Mo. High-resolution transmission electron microscopy was used to visualize a “core-shell” structure in the nanocrystalline fraction, which includes such phases as Ti1−nMonCxNy , Ni, NiO, TiO2, MoC0.5N0.5. The experimental data on measurements of the specific surface area by the BET method and the pycnomentric density made it possible to determine the calculated values of the average particle sizes which were 365 and 56 nm for the fractions from the cyclone and the filter, respectively. The average particle size of the nanocrystalline component of the fraction from the cyclone, according to the results of direct measurements, was 22 nm. Based on the obtained experimental results, a model for the formation of Ti0.8Mo0.2C0.5N0.5–Ni–Mo “core-shell” structures has been developed, which is implemented under the conditions of a turbulent flow of nitrogen gas formed in a quenching chamber of a plasma chemical plant.
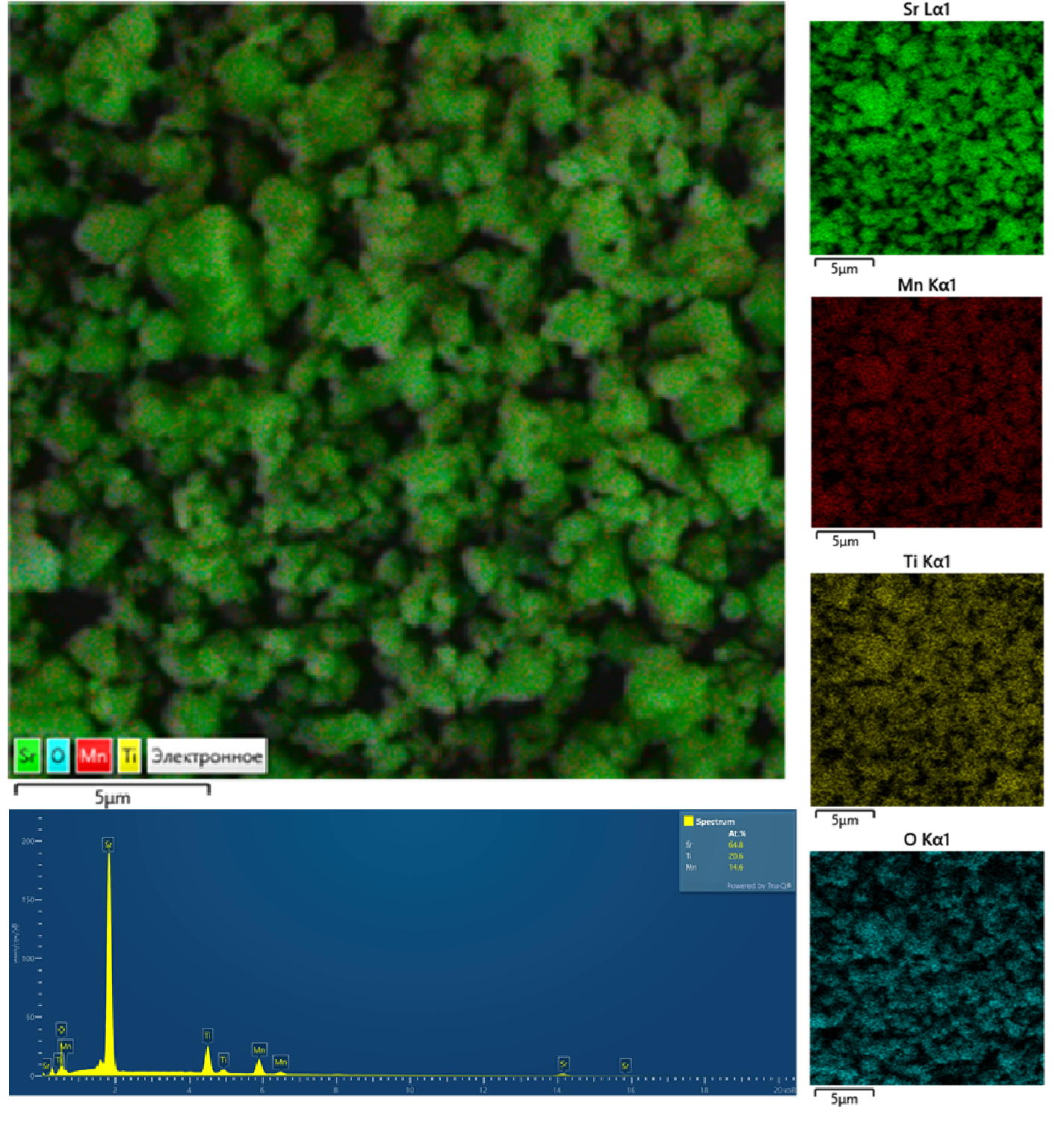
The photocatalytic properties of single-phase sample Sr2Mn0.4Ti0.6O4 is studied as a representative of a series of Sr2MnxTi1−xO4 solid solutions (x = 0.05, 0.15, 0.25, 0.4) obtained by the SHS. The sample annealed at 1200 ◦C is characterized by a uniform distribution of Sr, Ti and Mn in the oxidation degree (4+) in side the aggregates, the average size of which does not exceed 1 μm. According to UV-Vis-NIR spectroscopy data, a narrowing of the band gap of Sr2TiO4 from 3.16 to 1.8 eV is observed when it is doped with 40 mol% of manganese. This is due to the high photoactivity of Sr2Mn0.4Ti0.6O4 in the HQ oxidation reaction in UV and blue light.
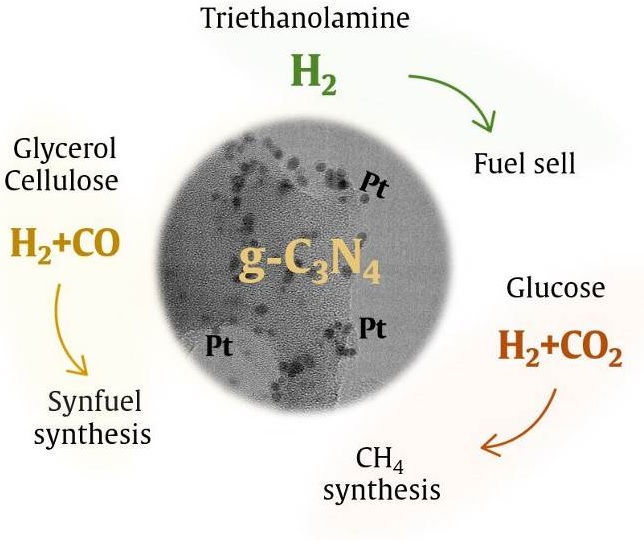
The results on the photocatalytic activity of 0 – 2 wt.% Pt/g-C3N4 in the hydrogen evolution reaction under visible light (430 nm) are presented. Triethanolamine (TEOA), glycerol, glucose and cellulose were used as electron donor. During the reaction, not only the target product, hydrogen, but also by-products of the reaction in the gas phase, namely CO and CO2, were controlled. In order to study the chemical composition, microstructure and optical properties, the samples were investigated by XPS, TEM and diffuse reflection methods. The maximum hydrogen evolution rate obtained for 1 % Pt/g-C3N4 from TEOA solution was 3.96 μmol·min−1, with a selectivity of 100 %. The use of glycerol and cellulose resulted in the production of syngas, and varying the platinum content allowed the selectivity of the process to vary (42.4 to 100 %). Glucose using led to the formation of a mixture of CO2 and H2 with a selectivity of 90 % or higher. In general, hydrogen-containing mixtures obtained using organic substrates can be further used in various applications.
ISSN 2305-7971 (Online)